Single Lane Sequencing is now available for NovaSeq X Plus! Visit our Sequencing of Customer-Prepared Libraries service page.
Blended Genome-Exome (BGE) Sequencing combines low-pass whole genome sequencing (WGS) and deeper coverage whole exome sequencing (WES), allowing high quality imputation of common variants across the genome as well as detection of rare coding variants across the exome region, from a single sample.
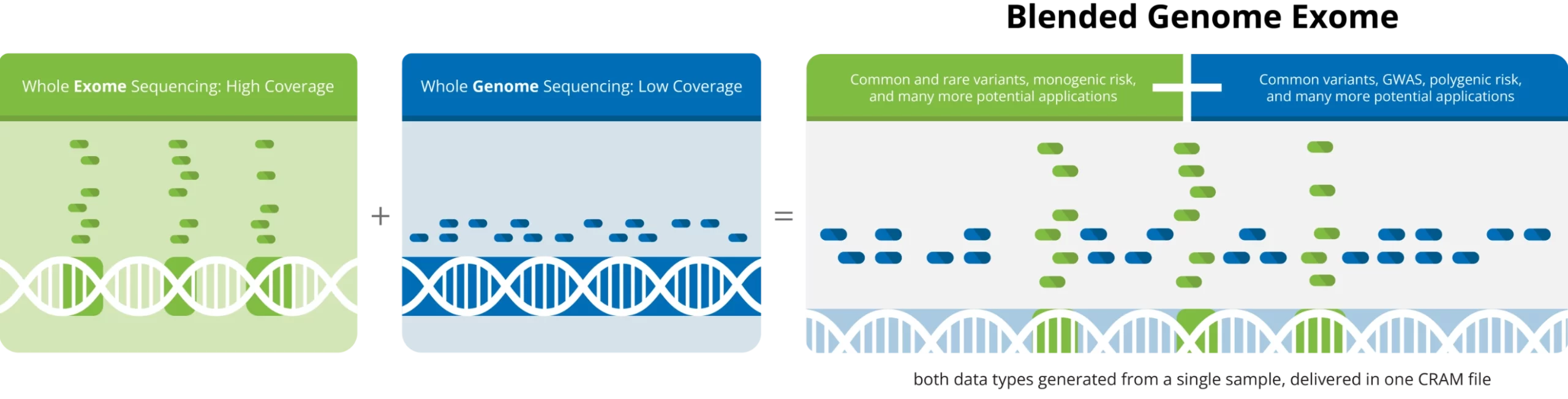
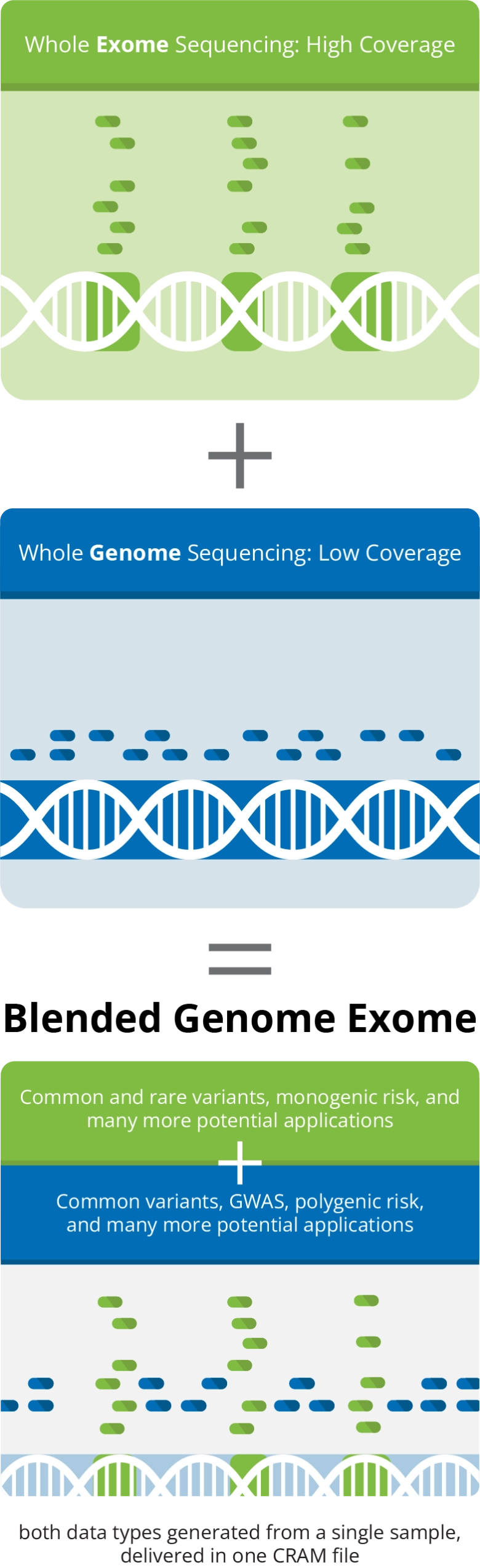
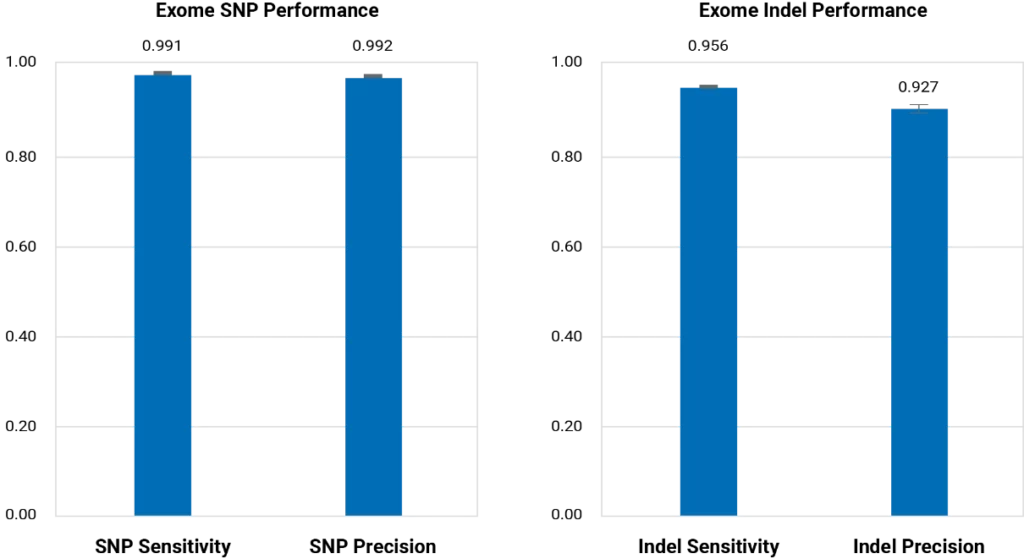
The unique BGE service is more cost effective than other options available for the assessment of both common and rare coding variants. With a capacity of 500,000 samples per year, we can readily accommodate a wide range of project sizes.
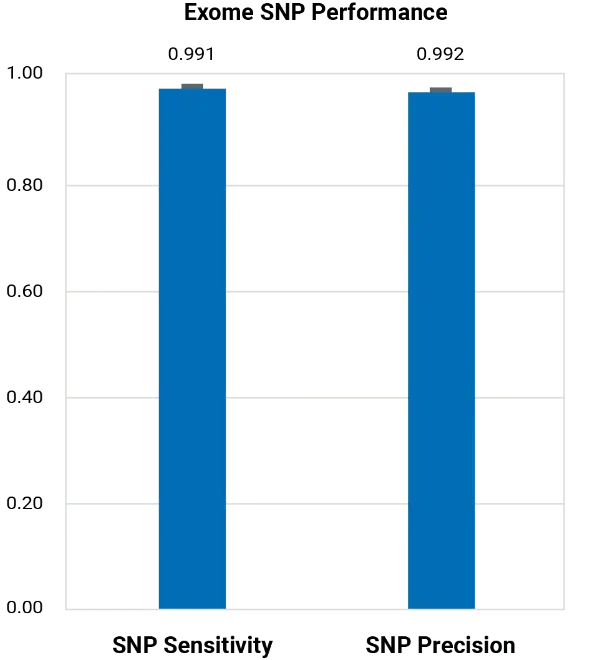
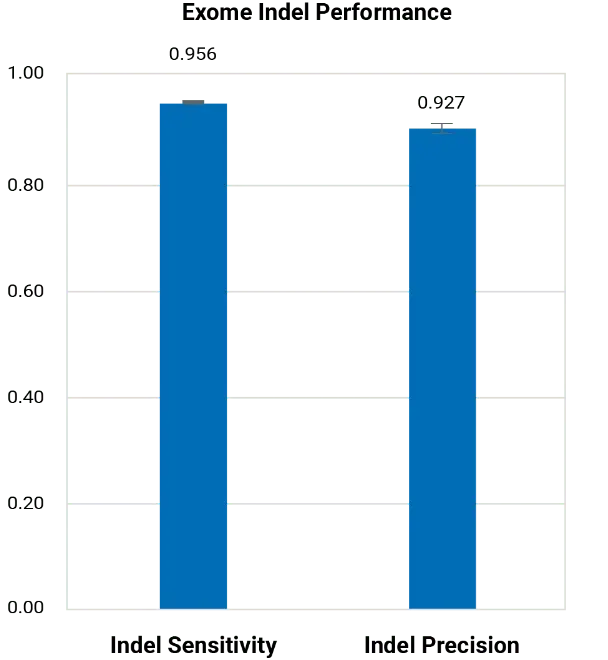
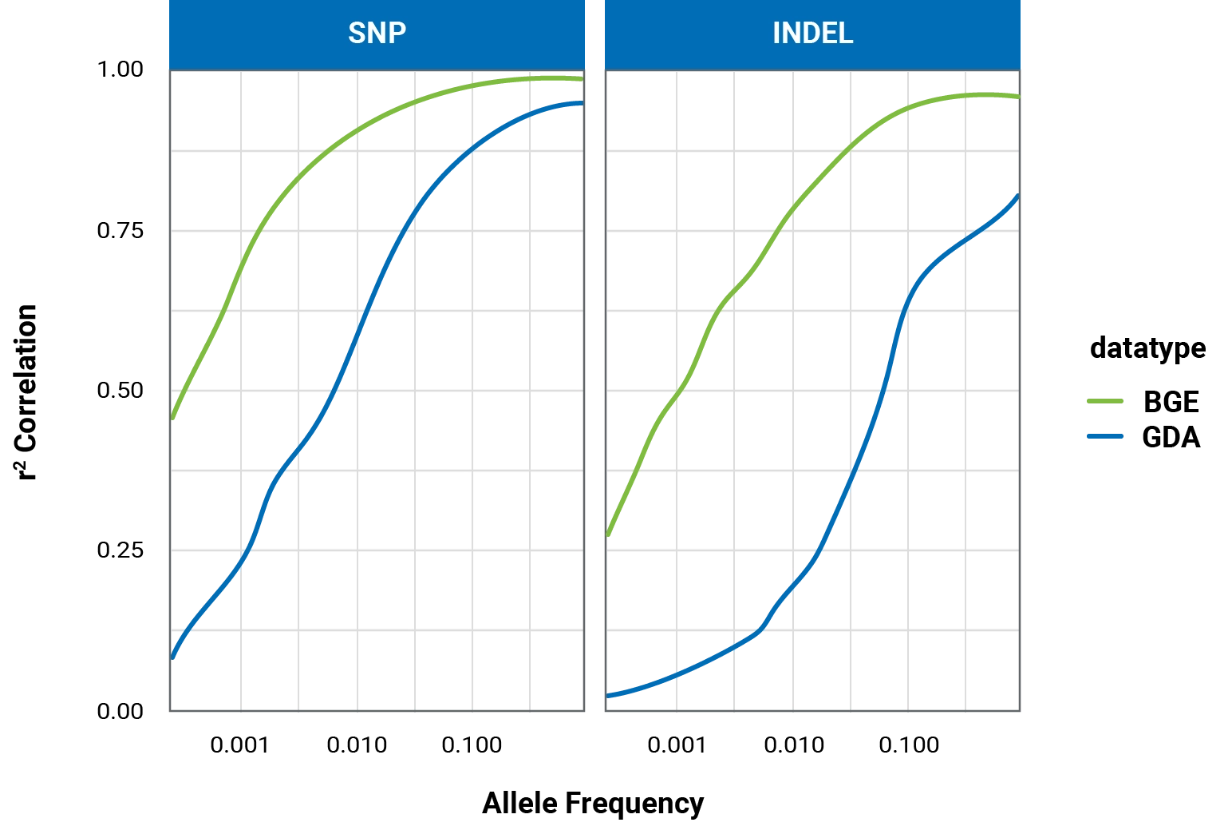
BGE outperforms commercial arrays for imputation across a range of allele frequencies and is far less biased, as population-specific variants rarely make it onto commercial arrays. BGE simultaneously achieves high sensitivity and precision for both SNP and Indel variant calling in the exome region.
Research Use
Genomic DNA: 15–110 ng/µL concentrations; 50–300 µL preferred volume, 30 µL minimum acceptable volume
Whole Blood, Saliva, Cell Pellet/Cell Suspension, Buccal Swab, Buffy Coat, Genomic DNA extracted from the aforementioned materials also accepted
≥90% of exome covered to ≥10X
≥9.5B PF aligned bases
Research Use
Genomic DNA: 15–110 ng/µL concentrations; 50–300 µL preferred volume, 30 µL minimum acceptable volume
Whole Blood, Saliva, Cell Pellet/Cell Suspension, Buccal Swab, Buffy Coat, Genomic DNA extracted from the aforementioned materials also accepted
≥90% of exome covered to ≥10X
≥9.5B PF aligned bases
| Category | Attribute | Minimum Threshold |
|---|---|---|
| Sample Quality Assessment | ||
| Library Quality/ Identity | Percent Mapped | ≥75% |
| Percent Contamination | ≤2.5% | |
| Genome Quality Assessment | ||
| Data Quality/ Quantity | Genome Mean Coverage | ≥1x |
| Exome Quality Assessment | ||
| Data Quality/Quantity | Percent Exome Bases at 20X | ≥90% |
| Exome Mean Target Coverage | ≥60x | |
| Variant Calling Quality | Percent Exome Callability | ≥95% |
| Category | Attribute | Minimum Threshold |
|---|---|---|
| Sample Quality Assessment | ||
| Library Quality/ Identity |
Percent Mapped | ≥75% |
| Percent Contamination | ≤2.5% | |
| Genome Quality Assessment | ||
| Data Quality/ Quantity | Genome Mean Coverage | ≥1x |
| Exome Quality Assessment | ||
| Data Quality/ Quantity |
Percent Exome Bases at 20X | ≥90% |
| Exome Mean Target Coverage | ≥60x | |
| Variant Calling Quality | Percent Exome Callability | ≥95% |
1
From a single sample, a PCR-free whole genome library is constructed and a sub-aliquot is taken through PCR amplification and exome selection.
2
Genome and exome libraries are blended together into one tube.
3
The blended sample is sequenced on the Illumina® NovaSeq X Plus platform, and a single CRAM file is generated containing low-coverage (1–3x) WGS and higher coverage (30–40x) WES data.
4


Regulatory Review
Quoting
Sample Kits
Inputs:
Blood
Saliva
DNA
One sample generates a whole exome and PCR-free whole genome library
The two libraries are pooled together to hit target coverage levels
Sequencing on Illumina® NovaSeq X Plus
Data (CRAM, VCF) delivered via secure cloud based platform
Copyright © 2025 Broad Institute. All Rights Reserved. Privacy Policy | Terms & Conditions
Chief of Clinical Strategy and Product Development, Broad Clinical Labs
Sean Hofherr is dual board certified by ABMGG in Clinical Biochemical Genetics and Clinical Molecular Genetics. Sean serves as the Chief of Clinical Strategy and Product Development at Broad Clinical Labs. In this role at BCL, Sean is able to leverage his extensive experience to guide the clinical vision and delivery across the organization. Sean most recently served as the Chief Operating Office at Fabric Genomics, which focuses on the use of AI and Bioinformatics for Clinical Interpretation of whole genome sequencing. Prior to Fabric, Sean was the Chief Scientific Officer and CLIA Director at the commercial reference laboratory, GeneDx.
Sean received his B.S. degree in Microbiology and Cell Sciences from the University of Florida before earning his Ph.D. in Molecular and Human Genetics from Baylor College of Medicine. Sean completed clinical fellowships in Clinical Biochemical Genetics and Clinical Molecular Genetics at the Mayo Clinic.
Chief of Staff, Broad Clinical Labs
As Broad Clinical Labs’ Chief of Staff, Danielle Perrin advises and supports colleagues on the executive leadership team in BCL’s strategic planning and execution. She builds and leads new organizational functions and processes and leads critical projects, as well as driving effective information flow, decision making, and execution throughout the organization. An operations leader with a business, engineering, and biology background and 20+ years of experience in the genomics field, Perrin has a track record of driving operational excellence and building and scaling both physical and business processes. During her career at Broad, which started in 2003 at the tail end of the Human Genome Project, Perrin has led laboratory operations and R&D teams in Broad’s Genomics Platform, as well as fulfilling senior advisory and leadership roles in the Broad Institute’s COO and CFO offices.
Perrin received her B.S. in Biology and M.E. in Biotechnology Engineering from Tufts University and her M.B.A. from the MIT Sloan School of Management.
Chief Commercial Officer, Broad Clinical Labs
As Chief Commercial Officer of Broad Clinical Labs, Tim De Smet leads BCL’s business development, alliance management, external project management, and customer support teams. A Broad Institute employee since 2008, De Smet has held leadership roles and managed teams of various sizes in Broad’s Genomics Platform and clinical lab, spanning laboratory operations, finance, and informatics, and has expertise in work design, financial modeling, and high scale laboratory and business operations.
De Smet received his B.S. in Biochemistry and M.B.A. from Northeastern University.
Chief Technology Officer, Broad Clinical Labs
As Chief Technology Officer, Jim Meldrim sets the vision for Broad Clinical Labs’ informatics systems, including the hardware and software used for sample intake and tracking, data production, analysis, and delivery. Having held a variety of laboratory and informatics-focused leadership roles at Broad, spanning R&D and production operations, Meldrim has been a leader and innovator in the generation, management, and analysis of genomic data since 1999, beginning with sequencing data generation for the Human Genome Project.
Meldrim received his B.S. in Biology from Cornell University.
Chief Operating Officer, Broad Clinical Labs
As Chief Operating Officer, Sheila Dodge leads Broad Clinical Labs’ process development and implementation activities, as well as lab operations, financial planning and operations, quality & compliance, and core business processes. A Six Sigma Black Belt with extensive experience in process development and high throughput genomics operations, Dodge is an expert in work design and in collaborating with a range of collaborators, scientists, engineers, and technology partners to rapidly integrate new technologies and operationalize innovations. A member of the Broad Institute since 2001, Dodge is an Institute Scientist and lectures at the MIT Sloan School of Management on operations, dynamic work design, and visual management techniques.
Dodge received her B.A. in biochemistry and molecular biology from Boston University and her master’s degree in biology from Harvard University. She earned her M.B.A. from MIT Sloan School of Management.
Chief Medical Officer and Clinical Laboratory Director, Broad Clinical Labs
Heidi Rehm is board-certified by ABMGG in Clinical Molecular Genetics and Genomics and serves as BCL’s Chief Medical Officer and Clinical Laboratory Director. She oversees BCL’s regulatory requirements, leads the clinical team performing genomic interpretation and variant analysis, and guides BCL’s efforts in genomic testing for clinical and research use. She is also an Institute Member of the Broad and co-director of the Medical and Population Genetics Program. Rehm is also the Chief Genomics Officer in the Department of Medicine and Genomic Medicine Unit Director at the Center for Genomic Medicine at Massachusetts General Hospital, working to integrate genomics into medical practice. She is a principal investigator of ClinGen, providing free and publicly accessible resources to support the interpretation of genes and variants. She co-leads both the Broad Center for Mendelian Genomics, focused on discovering novel rare disease genes, and the Matchmaker Exchange, which aids in gene discovery. She is Chair of the Global Alliance for Genomics and Health, a principal investigator of the Broad-LMM-Color All of Us Genome Center, co-leader of the Genome Aggregation Database (gnomAD), and a Board Member and Vice President of Laboratory Genetics for the American College of Medical Genetics and Genomics.
Rehm received her B.A. degree in molecular biology and biochemistry from Middlebury College before earning her M.S. in biomedical science from Harvard Medical School and Ph.D. in genetics from Harvard University. She completed her post-doctoral training with David Corey in neurobiology and a fellowship in clinical molecular genetics at Harvard Medical School.
Chair and Chief Scientific Officer, Broad Clinical Labs
As Chair and Chief Scientific Officer of Broad Clinical Labs, Niall Lennon leads the team and sets the scientific and clinical vision for the organization. Dr. Lennon joined the Broad Institute in 2006 and has since contributed to the development of applications for every major massively parallel sequencing platform across a range of fields. In 2013 Dr. Lennon led the effort to establish a CLIA licensed, CAP-accredited clinical laboratory at the Broad Institute to facilitate return of results to patients and to support clinical trials. More recently, he has led efforts to achieve FDA approval for large-scale genomics projects (NIH’s All of Us Research Program) and for Broad’s own clinical diagnostic for COVID-19 testing operation, which returned 37+ million results to patients. Dr. Lennon is a principal investigator of the eMerge and All of Us projects, an Institute Scientist at Broad, Associate Director of Broad’s Gerstner Center for Cancer Diagnostics, and an adjunct professor of biomedical engineering at Tufts University, where he teaches Molecular Biotechnology.
Dr. Lennon received a Ph.D. in pharmacology from University College Dublin and completed his postdoctoral studies at Harvard Medical School and Massachusetts General Hospital. He holds an executive certificate in management from the MIT Sloan School of Management.